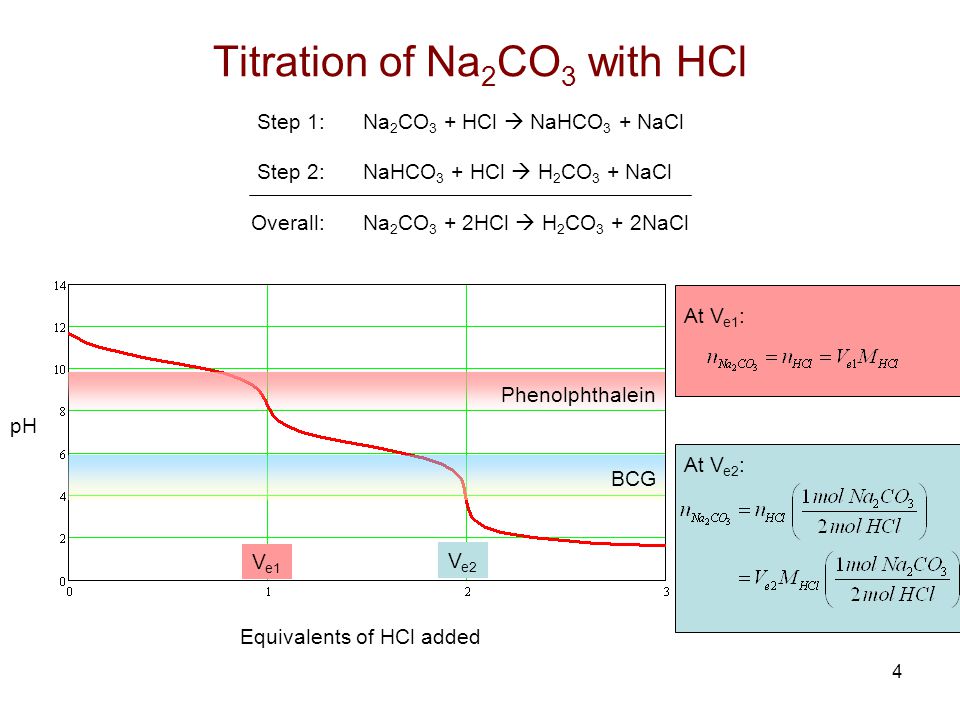
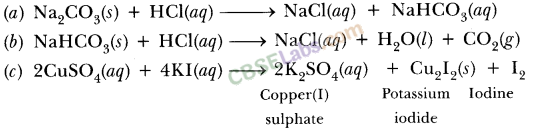In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with phenolphthalein indicator and y mL with methyl orange indicator in the same titration. Hence, volume for complete

Reaction of Hydrogen Chloride Gas with Sodium Carbonate and Its Deep Removal in a Fixed-Bed Reactor | Industrial & Engineering Chemistry Research

give inference for following test :- mixture+dil Hcl pass the evolved gas into NaCo3 phenolphthalein reagent - Brainly.in
What are reasons for getting two different concentration values of Na2CO3 when it was titrated with HCL using Phenolphthalein and Methyl Orange? - Quora
What volume of 0.25M Hcl is required to react completely with 22.6g of Na2co3 according to the equation Na2Co3+2Hcl=2Nacl=H20 (2) The molecular mass of organic compound is 78

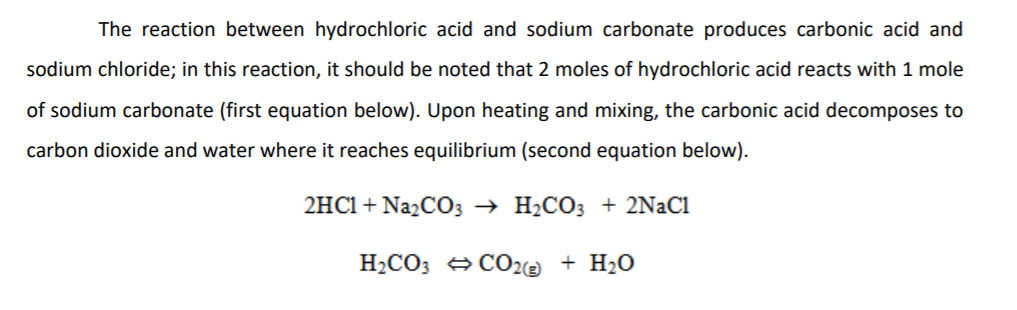
We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com
What are the products of na2co3 and HCL reaction when methyl orange and phenolphthalein are used as indicators? - Quora

Question Video: Determining the Products Formed from the Reaction between Sodium Carbonate and Hydrochloric Acid | Nagwa

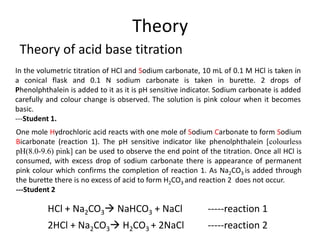
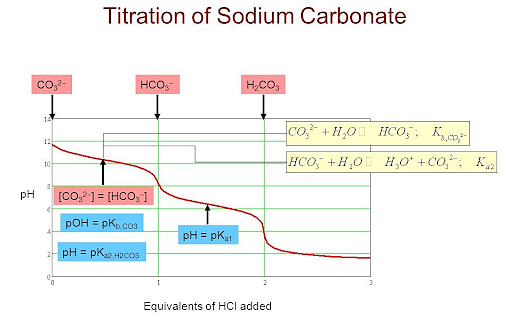
During the titration of sodium carbonate with H Cl, the dissolved carbonate ion will exist in three different forms; CO_3^{-2}, H CO_3^{-1}, and H_2 CO_3. During which part of the titration (initial,

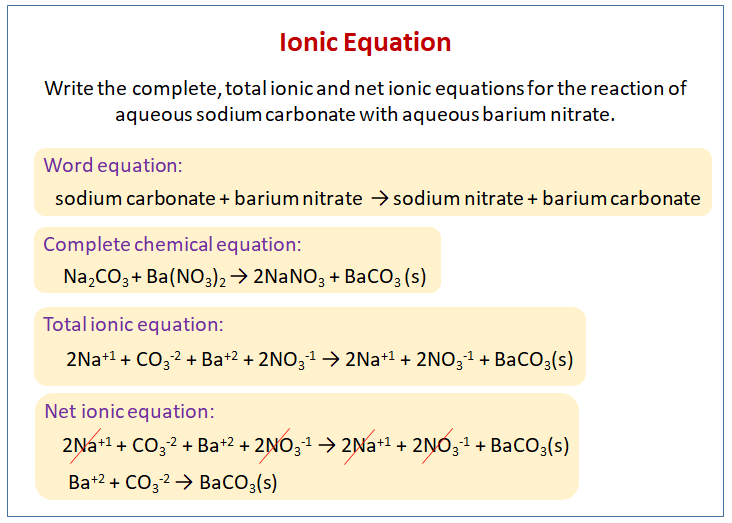
SOLVED: 2. Na2CO3(aq) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l) Balanced Equation: Complete Ionic Equation: Net Ionic Equation:

Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube

Question Video: Identifying the Observations of the Reaction between Hydrochloric Acid and Sodium Carbonate | Nagwa